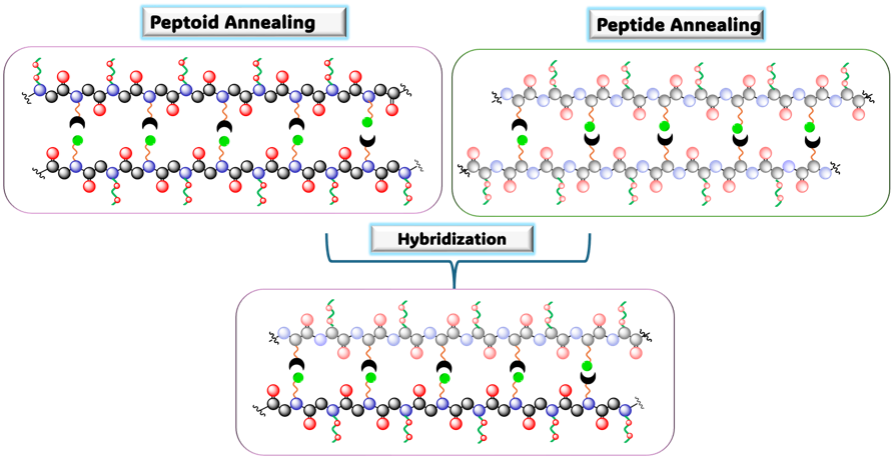
We seek to understand how the kinetics and thermodynamics of mixed TORC-mer duplexes depends on the backbone, using reasonably flexible systems: peptides, peptoids and urethanes
Peptoids, or poly-N-substituted glycines, are structural analogs to peptides that differ on where their side chains are attached. The peptoids backbone allows for a larger and more facile incorporation of different side chains. In our lab, we are interested in exploring the peptoid backbone as the carrier of TORC bonding pairs that will act as the encoders of synthetic “genetic” information, for the development of abiotic self-replicators.
Currently, we are conducting single-molecule Förster energy transfer analysis to further understand the kinetics that lead to the formation of self-assembled molecular ladders of peptoids bearing the amine/carbonyl TORC pair. Additionally, we are interested in exploring the annealing of peptoid-peptide TORC-bearing hybrid systems, to determine if there is backbone-induced selectivity.