Overview
The U.S. National Science Foundation Center for the Creation of Abiotic Replicating Materials and Assemblies (NSF CARMA) explores sequence-defined chemical chains using four types of chemical pairs that mimic biological structures but function differently. Researchers on the project study how these pairs can connect and disconnect, focusing on different molecular designs to improve the precision of this process. By understanding how these chemical bonds work, NSF CARMA aims to develop methods for effective replication and evolution of these materials in the future.
Three Project Aims:
Earlier Works
The team envisions ultimately developing new sustainable materials with the potential to grow, adapt to stress and respond to stimuli when given the proper ingredients or starting material. Already, scientific team members have achieved:
- Developing Tunable Orthogonal Reversible Covalent (TORC) bonds, that are now finding their way into a variety of materials, such as plastics that are remoldable yet also tough.
- Pioneering and developing self-healing materials capable of repairing themselves by making and breaking dynamic covalent bonds.
- Creating models that effectively predict the time required for a dynamic process to backtrack from errors involving misconnections.
Tunable Orthogonal Reversible Covalent (TORC)
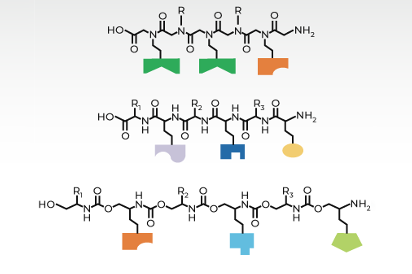
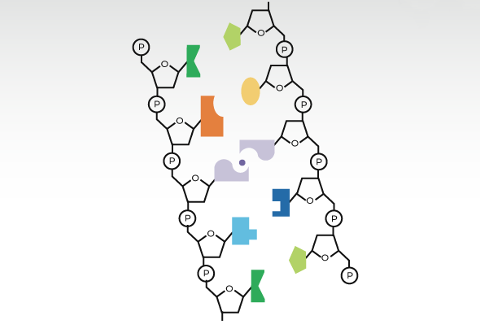
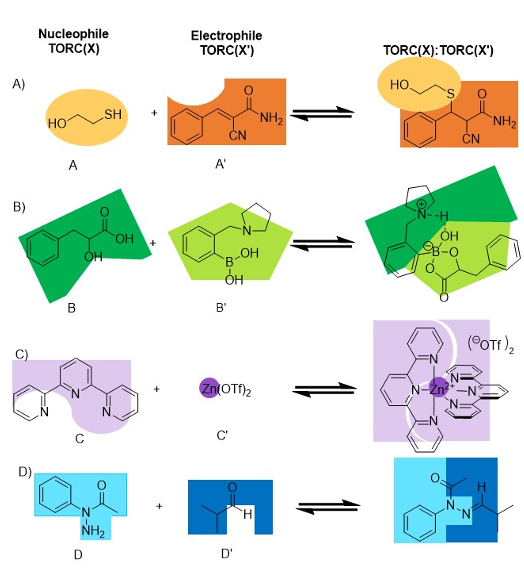
The NSF CARMA program centers around the use of Tunable Orthogonal Reversible Covalent (TORC) bonding pairs. Four classes of reactions are embodied by the TORC pairs: thiol conjugate additions, boronic acid / diol condensations, metal-chelation, and amine / carbonyl condensations. Each pair can be considered a combination of a nucleophile with an electrophile, as Figure 1 displays on the left and right, respectively.
For the acronym TORC, each term plays an essential role. Tunability (T) means we can change the structures within the general class of each of the four pairs of reactions with physical organic chemical insights, such as substituent effects to adjust their kinetics and thermodynamics. Importantly, pairing is orthogonal (O). As the T implies, we can adjust the structures within the pairs and retain the orthogonality.
The first pair (A:A') is an addition involving unsaturated-EWG (Electron Withdrawing Group) conjugate acceptor with thiols. The second pair (B:B') is a boronic acid/diol condensation. The third pair (C:C') simply requires ligands to the metals that do not react as nucleophiles with the electrophiles of the other three TORC pairs. Lastly, the fourth pair (D:D') is a condensation of an amine with an aldehyde.
This covalent (C) pairing is distinct from nature’s use of hydrogen bonding/base stacking. Nonetheless, the pairing is still reversible (R), albeit less dynamic than hydrogen-bonding. TORC pairs can be incorporated into strands analogous to, or drastically different than, nucleic acid duplexes.